Underground Storage Tanks offered key benefits for communities and industries when it comes to appearance, cost and security. They are also protected from the effects of fire. Mainly the reason why most of the owners preferred to install it underground.
With more owners and customers choosing to hide their propane tanks with popular underground installations, the need to protect steel vessels from moisture and chemicals in the soil has never been greater. UG tanks can rust and cause problems for the owners.
Last year, the Environment Department of Dubai Municipality had release a code for installation of Fuel storage Tank requiring each underground (UG) tanks installed must have a cathodic protection system or similar internationally recognised method to help prevent corrosion of the tank. There are also requirements for testing the effectiveness of the system on a specified schedule, to document the testing, and to maintain the test results for a specified time.
Why Do Underground Tanks Need Cathodic Protection?
• To minimize the rusting of steel tanks and connected piping which can cause a release of a regulated substance.
• To eliminate the cost associated with environmental clean-ups caused by a release from the UST system.
• To extend the operational life of the UG tank.
Preventing Corrosion
Protecting underground tanks from corrosion is easily achieved by the use of two commonly applied protection methods: external coating and cathodic protection. These two methods are complementary and should be used in conjunction with the other.
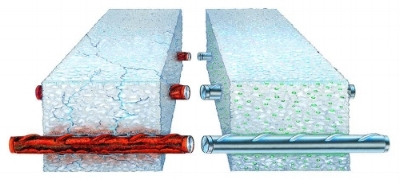
Cathodic protection prevents corrosion at those defects by applying DC current from an external source, forcing the tank to become cathode. Application of sufficient DC current to the tank will prevent any corrosion from occurring. The two general types of cathodic protection systems are sacrificial and impressed current. Sacrificial systems are used when the amount of current required for the protection is small, such as in underground propane tanks. Impressed current systems are more commonly used for large structures such as large diameter pipelines. Electrical isolation of the tank from metallic piping systems and electrical grounds is critical for the cathodic protection system’s effectiveness.
Sacrificial systems work by creating a galvanic connection between two different metals. The most common anode material is magnesium, which when coupled to steel results in DC current flow from the magnesium to the steel. The open circuit potential of steel is about -0.50 volts referenced to a copper sulfate electrode. The open circuit potential of magnesium is about -1.55V to -1.80V. By connecting the two metals together, the difference of 1 to 1.25V volts results in current flow to the tank that overcomes the natural corrosion cells that exist on the tank. With this current available to the tank, no corrosion occurs.